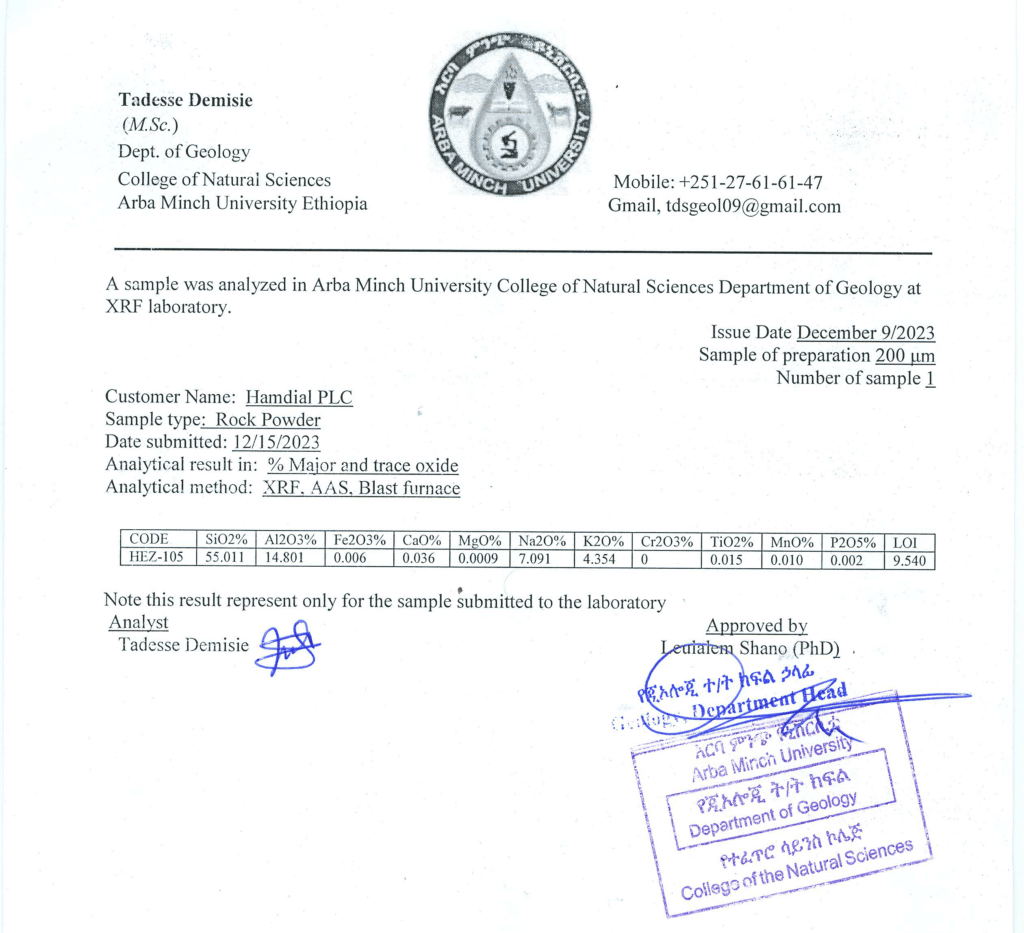
Zeolite
hamdail plc
Zeolite
Zeolite is a very versatile mineral. The internal honeycomb structure
and net negative charge can be used for all sorts of industrial and
scientific applications far beyond the common odor control products
for household animals.
Natural and synthetic zeolites are used commercially because of their
unique adsorption, ion-exchange, molecular sieve and catalytic
property.
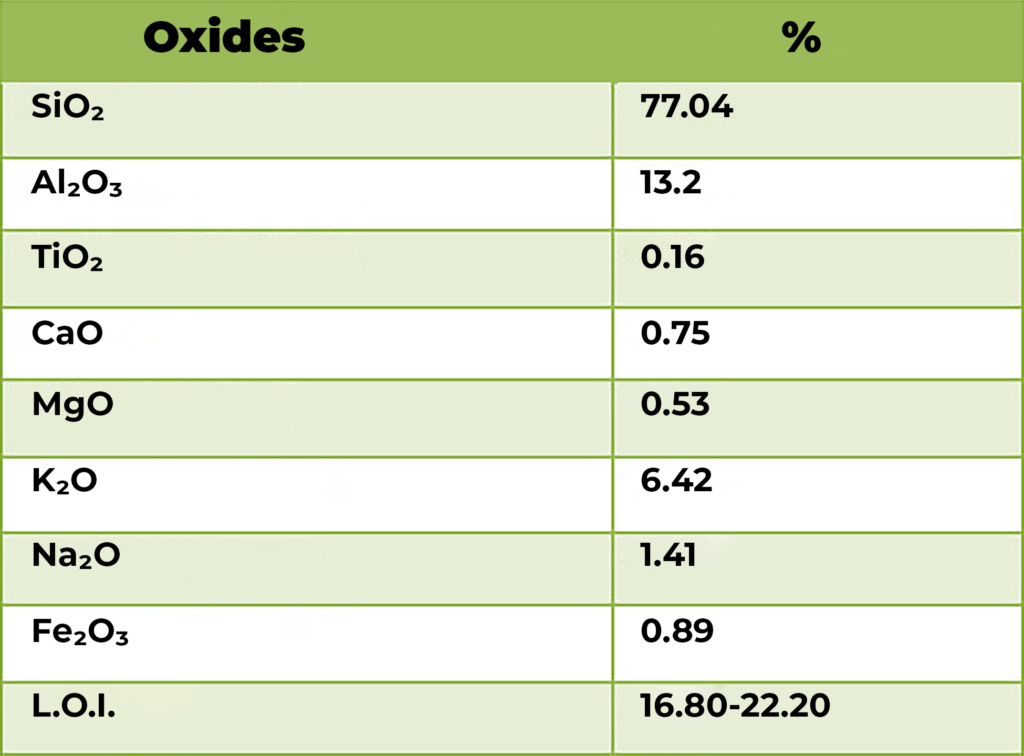
The general formula of Zeolite stands: MxAlxSi1−xO2·yH2O [here, M is
either a metal ion or H+]. Main constituents of Zeolite are Aluminum,
Silicon and Oxygen.

Applications
There are three main uses of zeolites in industry: catalysis, gas
separation and ion exchange.
Catalysis: Zeolites are extremely useful as catalysts for several
important reactions involving organic molecules. The most important
are cracking, isomerization and hydrocarbon synthesis. Zeolites can
promote a diverse range of catalytic reactions including acid-base and
metal induced reactions. Zeolites can also be acid catalysts and can be
used as supports for active metals or reagents.
The main industrial application areas are: petroleum refining, and
petrochemical production. Synthetic zeolites are the most important
catalysts in petrochemical refineries.
Adsorption: Zeolites are used to adsorb a variety of materials. This
includes applications in drying, purification, and separation. They can
remove water to very low partial pressures and are very effective
desiccants, with a capacity of up to more than 25% of their weight in
water. They can remove volatile organic chemicals from air streams,
separate isomers and mixtures of gases. A widely used property of
zeolites is that of gas separation.
Ion exchange: Hydrated cations within the zeolite pores are bound
loosely to the zeolite framework, and can readily exchange with other
cations when in aqueous media. Applications of this can be seen in
water softening devices, and the use of zeolites in detergents and
soaps. The largest volume use for zeolites is in detergent formulations
where they have replaced phosphates as water-softening agents. They
do this by exchanging the sodium in the zeolite for the calcium and
magnesium present in the water. It is even possible to remove
radioactive ions from contaminated water.
What makes zeolites notable is their crystalline structure perforated by
microscopic pores. These pores allow zeolites to act as natural filters.
Natural zeolite is a new and very good natural filter medium available
for the filtration of water. It offers superior performance to sand and
carbon filters, giving purer water and higher throughput rates with less
maintenance required. It has many advantages over sand and can be
used to directly replace sand in a normal sand filter.
The natural zeolite powder can be widely used in detergent industry
producing perfumed soap, laundry soap and whiten soap to improve
the outlook and washing.
From a practical point of view, zeolite can be adapted for a variety of uses:
Aquaculture:
Ammonia filtration in fish hatcheries
Bio-filter media
Agriculture:
Odor control
Confined animal environmental control
Livestock feed additives
Horticulture:
Nurseries, Greenhouses
Floriculture
Vegetables/herbs
Foliage
Tree and shrub transplanting
Turf grass soil amendment
Reclamation, revegetation, landscaping
Silviculture (forestry, tree plantations)
Medium for hydroponics growing
Household products:
Household odor control
Pet odor control
Industrial products:
Absorbents for oil and spills
Gas separations
Water treatment:
Water filtration
Heavy metal removal
Swimming pools
Wastewater treatment:
Ammonia/ammonium removal in municipal
sludge/wastewater
Heavy metal removal
Septic leach fields
